Expanding the Potential of CAR T Cell Therapy
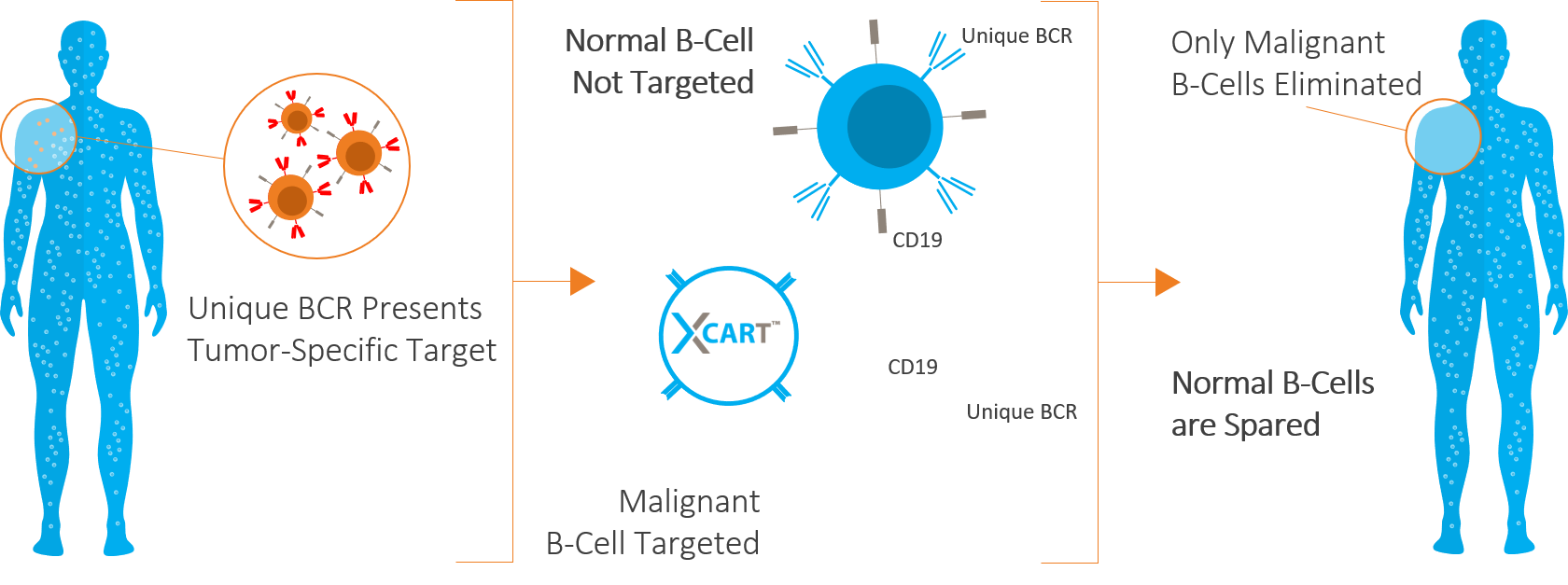
The XCART technology platform was designed to utilize an established screening technique to identify polypeptide domains that selectively bind to the unique B-cell receptor (BCR) on the surface of an individual lymphoma patient’s malignant B-cell clones. This BCR-selective targeting domain is engineered into the antigen-binding domain of a chimeric antigen receptor (CAR), creating the possibility of a CAR T treatment that should only recognize a given patient’s malignant B-cell clones. We believe our personalized CAR T therapies have the potential to offer cancer patients substantial benefits over the existing standard of care and currently approved CAR T therapies.
B-Cell Non-Hodgkin Lymphoma and Tumor Growth
B-Cell Non-Hodgkin's lymphoma is a type of cancer that originates in your lymphatic system, particularly the B-cells. When a tumor develops, it retains the unique B-Cell receptor (BCR) clone.

An expected result for XCART is limited off-tumor toxicities, such as B-cell aplasia. Xenetic’s clinical development program will seek to confirm the early preclinical results, and to demonstrate a more attractive safety profile than existing therapies.
 Only Targets Malignant B-Cells
Only Targets Malignant B-Cells
While currently approved therapies are designed to target the CD19 receptor that is common on all B-Cells, XCART is designed to target the tumor-specific antigens that are independent of CD19 or other antigens common to all B-Cells.